Aquaponics Health: Understanding Ammonia, Water Temperature and pH Balance
There is nothing worse in Aquaponics than to see your fish suddenly go belly up and die without any apparent reason.
People sometimes remark that their perfectly good fish that were fine yesterday – suddenly looked sick and died this morning.
They are left scratching their head – wondering what happened?
One point that many people overlook in Aquaponics is the relationship between Ammonia levels, Water Temperature and pH. There is a relationship between these three things that are hardly ever noticed by most people and if their inter-relationships are not understood – it can lead to impending disaster.
Lets have a closer look.
Ammonia
All fish give off ammonia. It comes off their gills and waste. Uneaten fish food turns into ammonia as it breaks down. If left to build up over time without nitrifying bacteria to convert it into Nitrates that the plants ingest – it will cripple your system and kill your fish. Ammonia is toxic to fish. Pure and simple. Fortunately we have our little friends – bacteria – doing a lot of the heavy lifting for us – fixing the imbalance. This is known as the Nitrogen Cycle, but it can take a new Aquaponics system some time to fully cycle. Six to eight weeks in winter or just a couple of weeks in Summer before the system is said to have “cycled” and all is in balance.
But at what point should you get worried about Ammonia levels becoming a threat to your fish given that ammonia is constantly being produced? The answer to this question will depend on the temperature of your tank water and the pH of your water, how heavily you stock your fish and how much uneaten fish food remains floating in the system. It gets more complicated with warmer water and a pH that is out of balance sitting at extreme levels.
What is pH?
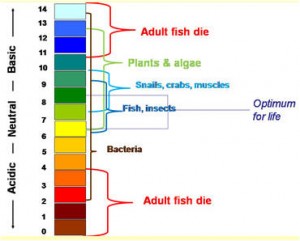
The pH is the measure of the hydrogen ion (H+) concentration in the water. The pH scale ranges from 0-14 with a pH of 7 being neutral. A pH below 7 is acidic and a pH of above 7 as basic. An optimal pH range for aquaponics is between 6 and 7 and can vary slightly depending on fish species.
Here at Ecofilms our tap water was a pH of 8 when we started out filling up our tank. Its too high for our fish. So when we first established our system we brought the pH down with a little acid. One or two tablespoons of hydrochloric acid (swimming pool acid) was needed to bring the pH down to the optimal level we want to grow fish and vegetables. Out tank in this case was near 1000 litres.
A pH between 6 and 7 is where we were aiming to bring the pH down to and we did it slowly over the course of a few days. Keeping pH at the correct level also prevents “nutrient lockout” enabling plants to readily take up the right nutrients and grow properly.
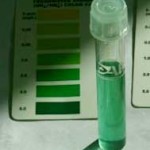
The pH was measured using an API Master test kit. Water from the fish tank is taken in a small glass vial and about 3 drops of the test chemical are added and measured against a colour chart. This chart will tell you on a scale where your pH is at. The same kit can also be used to tell you your Ammonia level. Ideally your Ammonia level should be near 0 but there will always be a trace level being emitted constantly by your fish. It gets more complicated if you stock your system with a heavy fish load.
Ammonia Toxicity and Water Temperature
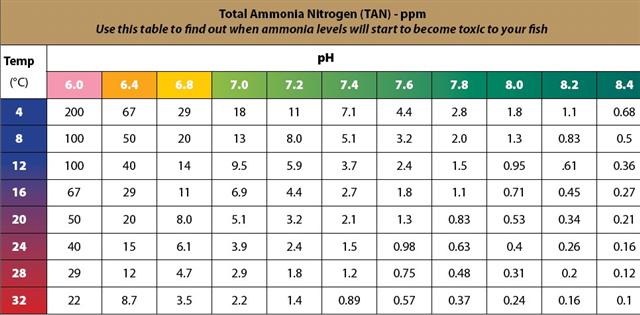
If you take a glance at the chart above you will notice the level of ammonia you can tolerate in your fish tank before it affects the fish. You will notice that at very warm water temperatures a small amount of ammonia can be toxic to your fish. At the opposite end of the spectrum in very cold water, the opposite is true. Fish can tolerate higher levels of ammonia the cooler the water. This also true for dissolved oxygen. Cold water can store more dissolved oxygen than the same volume of warm water. Something we should all be mindful of. Overstocking your tank with fish is fraught with danger if any of these parameters are pushed beyond their tolerable limits.
This lesson became very apparent for us at Ecofilms when we tried raising some small fry Barramundi in a heated tank over winter. A small spike in ammonia became toxic to the baby fish and we suffered some losses over the next few days before the system was stabilised. Our pH was sitting at around 8 and our water temperature was quite warm at 26 degrees centigrade. Barramundi love warm water but in this case it wasn’t enough to save some of them.
Here’s what we should have done:
- Make sure the system had cycled properly before introducing the fish
- Stop feeding the fish when ammonia levels were recorded to be high
- Brought the pH down to the mid to low 7’s over a number of days. The fish would have tolerated the lower pH.
- Done a partial water change (about a third) to reduce the amount of ammonia in the system and repeat and water test the system over the next few days.
Understanding the relationship between ammonia and water temperature will give you control over how well you can manage your system and avert danger if your water temperature suddenly climbs over summer and you fish begin to look weak and stressed.








Thanks – my fish today started dying. I had a ph of 7.8 and 1.0ppm of ammonia. I’ve just added some squeezed lemon and done a 2/3 water change. Have lost 5 so far, hopefully I’ve got there in time to not loose too many more. Thanks for your article.
Ditto’s here too, just getting involved with this amazing system, so this is really good to know early one.
Thanks,
Dave